Probiotics as a Harpoon for Targeting Oral Cancers: A Brief Update
Main Article Content
Abstract
Aim: To review different strains of probiotic bacteria and their role in the carcinogenesis process.
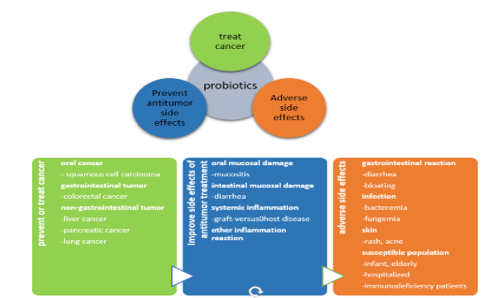
Background: Oral cancer is the sixth most prevalent malignancy in the world in terms of prevalence and death rates. Although oral cancer is treated most commonly with chemotherapy and immunotherapy, the formation of resistance to these treatments is a major downside that leads to disease recurrence. Probiotics are currently being advised as adjuvant and complementary therapeutic strategies for boosting the effects of chemotherapy and immunotherapy medications. Probiotics, or friendly microflora in our bodies, aid in the manufacture of beneficial compounds that protect the immune system from diseases such as cancer.
Review Results: In a detailed review, it was found that many probiotic strains of Lactobacillus species took an active participation in the management of oral cancers, leading to their decreased frequency in occurrence, along with few other probiotic species like Acetobacter syzygii and Bifidobacterium species.
Conclusion: Probiotics have anti-cancer properties that help in the prevention and inhibition of cancers, focusing mainly on oral cancers. They aid in the reduced occurrence of oral cancers, with the help of certain strains of the lactobacillus species mostly, by restoring the balance of the oral microbiota to its normal healthy state in various cases.
Clinical Significance: Oral probiotics can be used as a cure to manage dysbiosis in the oral environment as well as restore the normal function of the oral microbiome, in cases of head and neck cancers, during the processes of treatment and recovery. Therefore, further studies regarding the actual mode of action of probiotics in the disease progression and management of oral cancers should be investigated, so as to understand the functioning of the probiotics in its recovery.
Article Details
References
Vigneswaran, N., & Williams, M. D. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral and Maxillofacial Surgery Clinics of North America. (2014);26:123–141.
Zendeboodi, Fatemeh & Khorshidian, Nasim & Mortazavian, Amir & Cruz, Adriano. Probiotic: conceptualization from a new approach. Current Opinion in Food Science.2020;32.
Maria Carmen Collado, Miguel Gueimonde, Seppo Salminen, Probiotics in Adhesion of Pathogens: Mechanisms of Action. Bioactive Foods in Promoting Health,Academic Press. 2010;353-370
Kumar, Manoj & Kumar, Ashok & Nagpal, Ravinder & Mohania, Dheeraj & Behare, Pradip & Verma, Vinod & Kumar, Pramod & Poddar, Dev & Aggarwal, P.K. & Henry, Jeya & Jain, Shalini & Yadav, Hariom. Cancer-preventing attributes of probiotics: An update. International journal of food sciences and nutrition.2010; 61. 473-96.
Singh D, Khan MA, Siddique HR. Therapeutic implications of probiotics in microbiota dysbiosis: A special reference to the liver and oral cancers. Life Sci. 2021 Nov 15;285:120008.
Wan Mohd Kamaluddin WNF, Rismayuddin NAR, Ismail AF, Mohamad Aidid E, Othman N, Mohamad NAH, Arzmi MH. Probiotic inhibits oral carcinogenesis: A systematic review and meta-analysis. Arch Oral Biol. 2020 Oct;118:104855.
Legesse Bedada T, Feto TK, Awoke KS, Garedew AD, Yifat FT, Birri DJ. Probiotics for cancer alternative prevention and treatment. Biomed Pharmacother. 2020 Sep;129:110409.
Fernandes CF, Shahani KM. Anticarcinogenic and Immunological Properties of Dietary Lactobacilli. J Food Prot. 1990 Aug;53(8):704-710.
Gadelle, D., P. Raibaud, and E. Sacquet. 1985. B-Glucuronidase activities of intestinal bacteria determined both in vitro and in vivo in gnotobiotic rats. Appl. Environ. Microbiol. 49:682-685.
Dodds, K. L., and D. L. Collins-Thompson. 1984. Incidence of nitritedepleting lactic acid bacteria in cured meats and in starter cultures. J. Food Prot. 47:7-10.
Dodds, K. L., and D. L. Collins-Thompson. 1985. Characteristics of nitrite reductase activity in Lactobacillus lactis TS4. Can. J. Microbiol.31:558-562.
Goldin, B. R., and S. L. Gorbach. 1983. The effect of oral administration of Lactobacillus and antibiotics on intestinal bacterial activity and chemical induction of large bowel tumors. Dev. Ind. Microbiol.25:139-150.
Goldin, B. R., L. Swenson, J. Dwyer, M. Sexton, and S. L. Gorbach. 1980. Effect of diet and Lactobacillus acidophilus supplements on human fecal bacterial enzymes. J. Natl. Cancer Inst. 64:255-261.
Sinha, D. K. 1979. Development of a nonfermented acidophilus milk and testing its properties. Ph.D. Dissertation. The University of Nebraska-Lincoln.
Fernandes, C. F., K. M. Shahani, and M. A. Amer. 1987. Therapeutic role of dietary lactobacilli and lactobacillic fermented dairy products.FEMS Microbiol. Rev. 46:343-356.
Ayebo, A. D., K. M. Shahani, R. Dam, and B. A. Friend. 1982. Ion exchange separation of the antitumor component(s) of yogurt dialyzate. J. Dairy Sci. 65:2388-2390.
Reddy, G. V., B. A. Friend, K. M. Shahani, and R. E. Farmer. 1983. Antitumor activity of yogurt components. J. Food Prot. 46:8-11.
Lee, H. 1989. Effect of Lactobacillus acidophilus on colon carcinogenesis.Ph.D. Dissertation. The University of Nebraska-Lincoln.
Friend, B. A., R. E. Farmer, and K. M. Shahani. 1982. Effect of feeding and intraperitoneal implantation of yogurt culture cells on Ehrlich ascites tumor. Milchwissenschaft 37:708-710.
Kohwi, Y., K. Imai, Z. Tamura, and Y. Hashimoto. 1978. Antitumor effect of Bifidobacterium infantis in mice. Gann 69:613-618.
Bogdanov, I. G., P. G. Dalev, A. I. Gurevich, M. N. Kolosov, V. P. Mal'Kova, L. A. Plemyannikova, and I. B. Sorokina. 1975. Antitumor glycopeptides from Lactobacillus bulgaricus cell wall. FEBS Lett.57:259-261.
Hla, T., & Neilson, K. (1992). Human cyclooxygenase-2 cDNA. Proceedings of the National Academy of Sciences of the United States of America, 89, 7384–7388.
Menter, D. G., Shilsky, R. L., & Dubois, R. N. Cyclooxygenase-2 and cancertreatment: Understanding the risk should be worth the reward. Clinical CancerResearch. (2010);16:1384–1390.
Wang F, Jiang L, Liu AP, Guo XH, Ren FZ. Analysis of antigenotoxicity of Lactobacillus salivarius by high performance liquid chromatography. Chinese J Anal Chem 2008;36:740–4.
Kanojia D, Vaidya MM. 4-nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol 2006;42:655–67.
Zhang, M., Wang, F., Jiang, L., Liu, R., Zhang, L., Lei, X., et al. Lactobacillus salivarius REN inhibits rat oral cancer induced by 4-nitroquioline 1-oxide. Cancer Prevention Research. (2013);6:686–694
Chen CC, Lin WC, Kong MS, Shi HN, Walker WA, Lin CY, et al. Oral inoculation of probiotics Lactobacillus acidophilus NCFM suppresses tumour growth both in segmental orthotopic colon cancer and extraintestinal tissue. Br J Nutr 2012;107:1623–34.
Lan A, Bruneau A, Bensaada M, Philippe C, Bellaud P, Rabot S, et al. Increased induction of apoptosis by Propionibacterium freudenreichiiTL133 in colonic mucosal crypts of human microbiota-associated rats treated with 1,2-dimethylhydrazine. Br J Nutr 2008;100:1251–9.
Foo NP, Ou Yang H, Chiu HH, Chan HY, Liao CC, Yu CK, et al. Probiotics prevent the development of 1,2-dimethylhydrazine (DMH)-induced colonic tumorigenesis through suppressed colonic mucosa cellular proliferation and increased stimulation of macrophages.J Agric Food Chem 2011;59:13337–45.
Aghazadeh Z, Pouralibaba F, Yari Khosroushahi A. The prophylactic effect of Acetobacter syzygii probiotic species against squamous cell carcinoma. J Dent Res Dent Clin Dent Prospects. 2017 Fall;11(4):208-214.
Kaur, K., Topchyan, P., Kozlowska, A. K., Ohanian, N., Chiang, J., Maung, P. O., et al. (2018). Super-charged NK cells inhibit growth and progression of stem-like/poorly differentiated oral tumors in vivo in humanized BLT mice; effect on tumor differentiation and response to chemotherapeutic drugs. Oncoimmunology, 7, 1–14.
Hendler R, Zhang Y. Probiotics in the treatment of colorectal cancer. Medicines(Basel) 2018;5(3):101
Asoudeh-Fard, A., Barzageri, A., Dehnad, A., Bastani, S., Golchin, A., &Omidi, Y. Lactobacillus plantarum induces apoptosis in oral cancer KB cells through upregulationof PTEN and downregulation of MAPK signalling pathways. BioImpacts. (2017);7:193–198.
Morrison, D. K. MAP kinase pathways. Cold Spring Harbor Perspectives in Biology, (2012);4:a011254
Chu, E. C., & Tarnawski, A. S. (2004). PTEN regulatory functions in tumor suppression and cell biology. Medical Science Monitor, 10, 235–242.
Di Marzio L, Russo FP, D’Alo` S, et al. Apoptotic effects of selected strains of lactic acid bacteria on a human T leucemia cell line are associated with bacterial arginine deiminase and/ or sphingomyelinase activities. Nutr Cancer 2001;40:185–96.
Riccia, D. N. D., Bizzini, F., Perilli, M. G., Polimeni, A., Trinchieri, V., Amicosante, G.,et al. Anti-inflammatory effects of Lactobacillus brevis (CD2) on periodontal disease. Oral Diseases. (2007);13:376–385.
Sharma, A., Rath, G. K., Chaudhary, S. P., Thakar, A., Mohanti, B. K., & Bahadur, S. Lactobacillus brevis CD2 lozenges reduce radiation- and chemotherapy-induced mucositis in patients with head and neck cancer: A randomized double-blind placebo-controlled study. European Journal of Oral Cancer,. .(2011);48:875–881.
Zhao, X.; Yi, R.; Qian, Y.; Park, K.Y. Lactobacillus plantarum YS-3 prevents activated carbon-induced constipation in mice. J. Med. Food 2018, 21, 575–584.
Zhang, Z.J.; Feng, X.; Jiang, J.; Xiao, P. Influence of Pachyman on biosynthesis level of mice serum IgA, IgG and IgM. Chin. J. Immunol. 2013, 29, 1213–1215.
Chen, S.C.; Zhu, K.;Wang, R.; Zhao, X. Preventive effect of polysaccharides from the large yellow croaker swim bladder on HCl/ethanol induced gastric injury in mice. Exp. Ther. Med. 2014, 5, 316–322
Zhang, Z.J.; Feng, X.; Jiang, J.; Xiao, P. Influence of Pachyman on biosynthesis level of mice serum IgA, IgG and IgM. Chin. J. Immunol. 2013, 29, 1213–1215.
Liu B, Zhang J, Yi R, Zhou X, Long X, Pan Y, Zhao X. Preventive Effect of Lactobacillus fermentum CQPC08 on 4-Nitroquineline-1-Oxide Induced Tongue Cancer in C57BL/6 Mice. Foods. 2019 Mar 11;8(3):93.
Kim SJ, Kim JK, Lee DU, Kwak JH and Lee SM: Genipin protects lipopolysaccharide induced apoptotic liver damage in D galactosamine sensitized mice. Eur J Pharmacol 635: 188 193, 2010. 3.
Zhu H, Yin R, Han F, Guan J, Zhang X, Mao X, Zhao L, Li Q, Hou X and Bi K: Characterization of chemical constituents in Zhi Zi Da Huang decoction by ultra high performance liquid chromatography coupled with quadrupole time of flight mass spectrometry. J Sep Sci 37: 3489 3496, 2014
Gong G, Zheng Z, Liu H, Wang L, Diao J, Wang P and Zhao G: Purification and characterization of a β glucosidase from asper¬gillus niger and its application in the hydrolysis of geniposide to genipin. J Microbiol Biotechnol 24: 788 794, 2014
Koo HJ, Song YS, Kim HJ, Lee YH, Hong SM, Kim SJ, Kim BC, Jin C, Lim CJ and Park EH: Antiinflammatory effects of genipin, an active principle of gardenia. Eur J Pharmacol 495: 201 208, 2004.
Cho HI, Kim SJ, Choi JW and Lee SM: Genipin alleviates sepsis induced liver injury by restoring autophagy. Br J Phar-macol 173: 980 991, 2016.
Cheng Z, Xu H, Wang X, Liu Z. Lactobacillus raises in vitro anticancer effect of geniposide in HSC-3 human oral squamous cell carcinoma cells. Exp Ther Med. 2017 Nov;14(5):4586-4594.
Mishra S, Rath S, Mohanty N. Probiotics-A complete oral healthcare package. J Integr Med. 2020;18(6):462-469.